Paxlovid Rebound Covid Phenomena
Pfizer’s new COVID drug, Paxlovid, was approved under emergency use authorization in December of 2021. This drug was reviewed in a previous blog post.
Paxlovid has gained attention in the past month due to a strange phenomenon happening among some people taking it. The government is now planning a study to investigate. What is happening with this new medication?
Paxlovid Refresher
Paxlovid is for use in non-hospitalized high-risk adults with mild to moderate COVID symptoms or exposure and given twice daily for 5 days.1
It is a combination of PF-07321332 and ritonavir, an antiretroviral protease inhibitor used in HIV/AIDS.1,2 Protease inhibitors are pharmaceuticals that bind and block enzyme activity necessary for replication of the virus.3,4 It is not anything like Ivermectin!
Paxlovid Long Term Safety
There is no long-term safety data for Paxlovid. The following is taken directly from Pfizer’s website and the drug fact sheet:5,6
- There are limited clinical data available for PAXLOVID. Serious and unexpected adverse events may occur that have not been previously reported with PAXLOVID use.
- The concomitant use of PAXLOVID and certain other drugs may result in potentially significant drug interactions.
- Hypersensitivity and anaphylactic reactions have been reported with PAXLOVID.
- Hepatotoxicity: Hepatic transaminase elevations, clinical hepatitis, and jaundice have occurred in patients receiving ritonavir.
- PAXLOVID may lead to a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection.
- Carcinogenicity studies have not been conducted with nirmatrelvir.
COVID Rebound After Paxlovid
A pre-print study7 was published describing this rebound phenomenon in a patient who had been treated with Paxlovid. A fully vaccinated and boosted 71-year-old male had a high exposure to COVID with symptoms. Day 0 he began a course of Paxlovid. His symptoms quickly and completely resolved by Day 2.
While continuing to isolate, the patient’s symptoms returned on Day 9 and peaked on Day 10. Viral antigen testing was conducted and compared from Day 1 and Day 9, showing two distinct peaks of the viral load. The following image shows those two peaks during Paxlovid treatment:
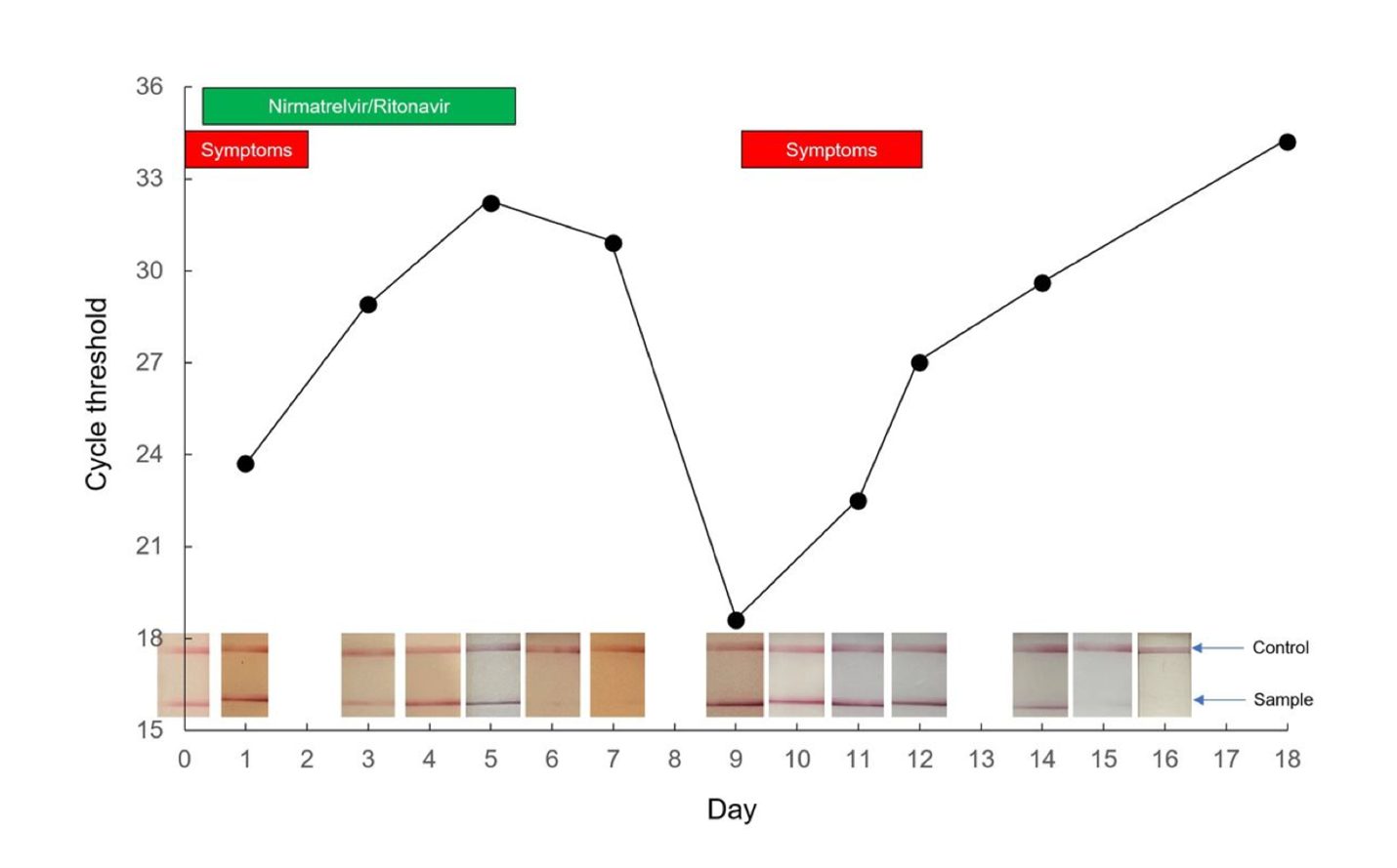
The author concludes that their findings suggest early treatment with Paxlovid “may transiently suppress viral replication before natural immunity is sufficient to complete the clearance of SARS-CoV-2.”
Post-Paxlovid Rebound Was Demonstrated in Pfizer Trials
The post-Paxlovid rebound phenomenon is not so surprising as it was observed by Pfizer in their clinical trial. Pfizer reported this data to the FDA review to approve it for emergency use. This information was briefly included on the 23rd page of the FDA document,8 stating the following:
“Several subjects appeared to have a rebound in SARS-CoV-2 RNA levels around Day 10 or Day 14, although this occurred among subjects with or without potential resistance-associated substitutions detected at Day 1 or Day 5.”
Just as seen in the pre-print image above, the same is shown in the following image from the FDA review document. As you can see, the lines suddenly shift in an upward trend between Days 10-14, representing the rebound or COVID relapse.
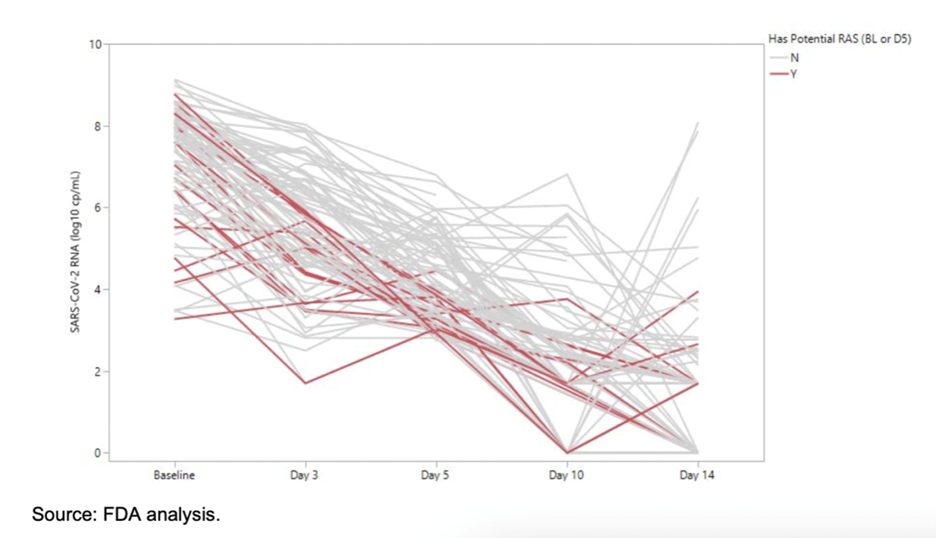
Though this phenomenon was reported to the FDA for review, the rebound information was not included in Pfizer’s New England Journal of Medicine paper, and therefore, was not made readily available to the public .9
Conclusion
Post-Paxlovid COVID rebound or relapse is now a documented phenomenon that is occurring among some people. While we know this occurs, there is much unknown. Pending the government’s investigation into this phenomenon, questions remain:
- How often does relapse occur?
- Does Paxlovid blunt the natural immune response?
- Are relapsed patients contagious during the second peak?
- Does resistance develop to Paxlovid?
- Should high risk individuals with relapse be treated again?
- What is the long-term safety?
We know that early treatment is critical when it comes to COVID, but does early treatment with Paxlovid come at a cost?
Have an awesome day! Dr D
-
https://www.drugs.com/history/paxlovid.html
-
https://pubchem.ncbi.nlm.nih.gov/compound/Ritonavir
-
https://www.ncbi.nlm.nih.gov/BOOKS/NBK548887/
-
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4396582/
-
https://www.covid19oralrx-hcp.com/safety
-
https://www.covid19oralrx-hcp.com/files/Clean_Emergency-Use_Full-Prescribing-Info_HCP-Fact-Sheet-COVID-19-oral-antiviral.pdf
-
https://assets.researchsquare.com/files/rs-1588371/v1/48342d2c-b3ea-4228-b600-168fca1fded7.pdf?c=1650977883
-
https://www.fda.gov/media/155194/download
-
https://www.nejm.org/doi/full/10.1056/NEJMoa2118542
